Liquid cooling
Liquid Cooling
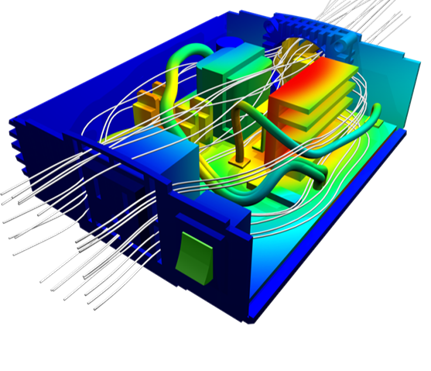
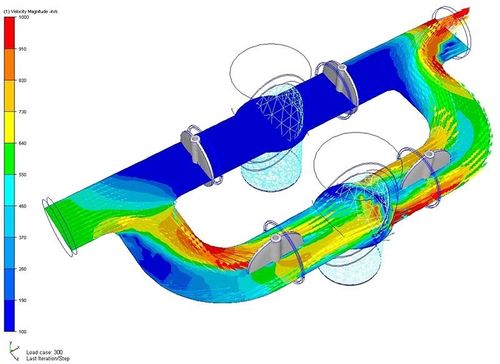
In brief : Liquid Cooling is a technology to reduce heat in electronic and mechanical devices through exploiting the properties of liquids. In science, liquid cooling is a combinded technology of Fluid Dynamics, Thermodynamics and Aerodynamics. In computer, the most common form of liquid cooling involves a closed system of tubes that carries the liquid from one component involved in cooling to another. These systems are generally referred to as loops. There are some parts common to all liquid cooling loops: pumps, tubing, water blocks, and radiators.
Fluid Dynamics
Fluid dynamics is an applied science that is concerned with the movement of liquids and gases. Fluid dynamics is one of two branches of fluid mechanics, which is the study of fluids and how forces affect them. Fluid dynamics provides methods for studying the evolution of stars, ocean currents, weather patterns, plate tectonics and even blood circulation. Some important technological applications of fluid dynamics include rocket engines, wind turbines, oil pipelines, air conditioning systems and liquid cooling on high performance computer system.
Thermodynamics
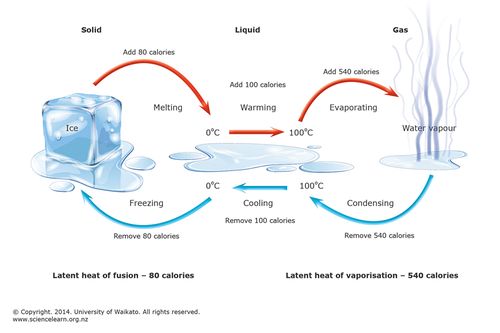
Thermodynamics is a study of the laws governing the transformation of heat energy to and from other forms of energy, thus of the efficient design and working of heat engines (such as the gas engine and the steam engine). Its 3+1 laws are as follows:
- First law: No form of energy can be created or destroyed but may be transformed from one to another. Therefore the total energy in the universe remains the same.
- Second law: It is impossible for heat to travel from an object at a lower temperature to another object at a higher temperature. Stated by the German physicist Rudolf Clausius (1822-1888) in two parts as, "Heat cannot be transferred from one body to a second body at a higher temperature without producing some effect," and "The entropy of a closed system increases with time."
- Third law: It is impossible to reduce any system to a level of absolute temperature or 0 Kelvin (-273.15°C or -459.67°F).
- Zeroth law of thermodynamics (that underlies all the above laws): If two systems are in thermal equilibrium with a third system, all three systems are in thermal equilibrium with one another.
Read more: http://www.businessdictionary.com/definition/thermodynamics.html
Thermodynamics-its system and processes
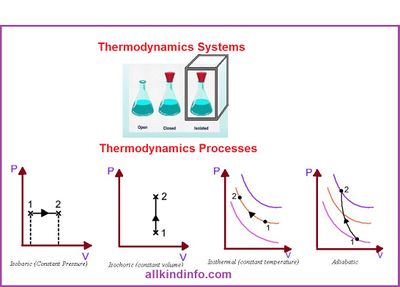
A definite quantity of matter bounded by some closed surface is known as a system and anything outside the system which can exchange energy with the system has a direct bearing on the behavior of the system is called surrounding
In thermodynamics, there are three common systems,
- Isolated Systems
- If a system is not influenced in any way by it’s surrounding, it is said to be isolated”. An isolated system exchanges neither matter nor energy with its surroundings. e.g. tea in a thermos flask
- Closed Systems
- System which can exchange only energy (not matter) with surrounding are known as a closed system” e.g. tea in a cup covered with a lid.
- Open Systems
- Systems which can exchange both matter & energy with surrounding are known as open systems” e.g. tea in a cup.
Aerodynamics
Aerodynamics is the way air moves around things. The rules of aerodynamics explain how an airplane is able to fly. Anything that moves through air reacts to aerodynamics. A rocket blasting off the launch pad and a kite in the sky react to aerodynamics. Aerodynamics even acts on cars, since air flows around cars and fans in high performance computers.